Introduction
Early stages of psychotic disorder have been regarded as critical periods for early intervention to improve the clinical outcome of the disorder (Correll et al., Reference Correll, Galling, Pawar, Krivko, Bonetto, Ruggeri and Kane2018; Fusar-Poli, McGorry, & Kane, Reference Fusar-Poli, McGorry and Kane2017; Lieberman et al., Reference Lieberman, Perkins, Belger, Chakos, Jarskog, Boteva and Gilmore2001). It has been well studied that a shorter duration of untreated psychosis (DUP) is related to a better prognosis of first-episode psychosis (FEP) patients (Marshall et al., Reference Marshall, Lewis, Lockwood, Drake, Jones and Croudace2005; Perkins, Gu, Boteva, & Lieberman, Reference Perkins, Gu, Boteva and Lieberman2005). In addition, the concept of clinical high risk (CHR) for psychosis was established not only for early detection of FEP to shorten the DUP but also for delaying or preventing the onset of psychotic disorder (Miller et al., Reference Miller, McGlashan, Rosen, Somjee, Markovich, Stein and Woods2002; Yung et al., Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio and Buckby2005). However, those patients in the early stages of psychotic disorder were proven to be heterogeneous in their prognostic trajectories. While approximately 40% of FEP patients show favorable outcomes, such as full remission or a partial response, the remaining patients display chronic, relapsing disease courses and are even treatment resistant (Birchwood, Todd, & Jackson, Reference Birchwood, Todd and Jackson1998; Lieberman, Reference Lieberman1993). Similarly, three prognostic trajectories of positive, moderately impaired, and severely impaired outcomes were found among CHR individuals through group-based multitrajectory modeling (Allswede et al., Reference Allswede, Addington, Bearden, Cadenhead, Cornblatt, Mathalon and Cannon2020). Meta-analytic studies reported that 22% of CHR subjects transitioned to a psychotic disorder within 3 years and 65% of CHR individuals were nonremitters within 1.94 years (Fusar-Poli et al., Reference Fusar-Poli, Bechdolf, Taylor, Bonoldi, Carpenter, Yung and McGuire2013, Reference Fusar-Poli, de Pablo, Correll, Meyer-Lindenberg, Millan, Borgwardt and Arango2020; Simon et al., Reference Simon, Borgwardt, Riecher-Rossler, Velthorst, de Haan and Fusar-Poli2013), suggesting that improving the general clinical outcome is as important as preventing the onset of psychotic disorder (Carrion et al., Reference Carrion, McLaughlin, Goldberg, Auther, Olsen, Olvet and Cornblatt2013; Lin et al., Reference Lin, Wood, Nelson, Beavan, McGorry and Yung2015; Schlosser et al., Reference Schlosser, Jacobson, Chen, Sugar, Niendam, Li and Cannon2012).
Prognostic heterogeneity produces significant difficulties in clinical decision making, such as decisions concerning the early use of clozapine in FEP patients who are resistant to usual antipsychotic treatment. Intervention for CHR individuals also involves challenges, such as deciding who should receive rigorous or less intensive interventions, including the justification of the use of antipsychotic medication. By using biological predictors that will aid in forecasting prognostic trajectories of early psychosis patients, patients’ suffering during trial and error regarding treatment will be resolved, and a significant amount of time and resources will be saved. Previous studies reported that cortical gyrification (Palaniyappan et al., Reference Palaniyappan, Marques, Taylor, Handley, Mondelli, Bonaccorso and Dazzan2013), bilateral hippocampal increase (Lappin et al., Reference Lappin, Morgan, Chalavi, Morgan, Reinders, Fearon and Dazzan2014), corticostriatal functional connectivity (Oh, Kim, Kim, Lee, & Kwon, Reference Oh, Kim, Kim, Lee and Kwon2020; Sarpal et al., Reference Sarpal, Argyelan, Robinson, Szeszko, Karlsgodt, John and Malhotra2016), glutathione and glutamate levels (Dempster et al., Reference Dempster, Jeon, MacKinley, Williamson, Theberge and Palaniyappan2020), and electrophysiological markers (Lho, Kim, Lee, Kwak, & Kwon, Reference Lho, Kim, Lee, Kwak and Kwon2019; Mi et al., Reference Mi, Wang, Li, She, Li, Huang and Zheng2021; Renaldi et al., Reference Renaldi, Kim, Lee, Kwak, Tanra and Kwon2019) were associated with the treatment response of FEP patients. In CHR subjects, structural brain imaging (Cannon et al., Reference Cannon, Chung, He, Sun, Jacobson and van Erp2015; de Wit et al., Reference de Wit, Ziermans, Nieuwenhuis, Schothorst, van Engeland, Kahn and Schnack2017; Ho et al., Reference Ho, Holt, Cheung, Iglesias, Goh, Wang and Zhou2017; Koutsouleris et al., Reference Koutsouleris, Riecher-Rossler, Meisenzahl, Smieskova, Studerus, Kambeitz-Ilankovic and Borgwardt2015; Koutsouleris, Upthegrove, & Wood, Reference Koutsouleris, Upthegrove and Wood2019; Reniers et al., Reference Reniers, Lin, Yung, Koutsouleris, Nelson, Cropley and Wood2017), neurochemical markers (Allen et al., Reference Allen, Chaddock, Egerton, Howes, Barker, Bonoldi and McGuire2015; Bossong et al., Reference Bossong, Antoniades, Azis, Samson, Quinn, Bonoldi and McGuire2019; Egerton et al., Reference Egerton, Stone, Chaddock, Barker, Bonoldi, Howard and McGuire2014), and event-related potential (ERP) markers (Bodatsch et al., Reference Bodatsch, Ruhrmann, Wagner, Muller, Schultze-Lutter, Frommann and Brockhaus-Dumke2011; Hamilton et al., Reference Hamilton, Roach, Bachman, Belger, Carrion, Duncan and Mathalon2019; Kim, Lee, Lee, Kim, & Kwon, Reference Kim, Lee, Lee, Kim and Kwon2015; Kim, Lee, Yoon, Lee, & Kwon, Reference Kim, Lee, Yoon, Lee and Kwon2018; Perez et al., Reference Perez, Woods, Roach, Ford, McGlashan, Srihari and Mathalon2014) were suggested as biological predictors of symptomatic and functional outcomes. However, clinically efficient biomarkers that forecast prognostic trajectories across the course of psychotic disorders, from CHR to FEP, have not yet been studied.
Mismatch negativity (MMN) is an ERP component that is elicited when repetitive standard stimuli are interrupted by infrequent deviant stimuli and is thus thought to be reflective of the automatic auditory change detection process (Naatanen & Escera, Reference Naatanen and Escera2000). Because MMN generation is associated with neurotransmission at the N-methyl-D-aspartate (NMDA) receptor, MMN has been widely studied across the course of psychotic disorders to elucidate the pathophysiological mechanism of schizophrenia (Javitt & Freedman, Reference Javitt and Freedman2015; Javitt, Steinschneider, Schroeder, & Arezzo, Reference Javitt, Steinschneider, Schroeder and Arezzo1996; Uno & Coyle, Reference Uno and Coyle2019). Reduced duration deviant MMN (dMMN) amplitude has been consistently reported in schizophrenia and early psychosis patients, including FEP patients and CHR individuals, although the degree of dMMN impairment is less significant than in chronic schizophrenia patients (Erickson, Ruffle, & Gold, Reference Erickson, Ruffle and Gold2016; Haigh, Coffman, & Salisbury, Reference Haigh, Coffman and Salisbury2017; Hamilton, Boos, & Mathalon, Reference Hamilton, Boos and Mathalon2020; Kim, Cho, Yoon, Lee, & Kwon, Reference Kim, Cho, Yoon, Lee and Kwon2017; Nagai et al., Reference Nagai, Tada, Kirihara, Yahata, Hashimoto, Araki and Kasai2013; Tateno et al., Reference Tateno, Higuchi, Nakajima, Sasabayashi, Nakamura, Ueno and Suzuki2021). Although little is known about dMMN as a prognostic predictor of FEP patients (Higgins, Lewandowski, Liukasemsarn, & Hall, Reference Higgins, Lewandowski, Liukasemsarn and Hall2021; Lho et al., Reference Lho, Kim, Park, Hwang, Moon, Oh and Kwon2020), previous studies, including our own, showed that dMMN was predictive of a transition to psychotic disorder, remission, and symptomatic and functional improvement in CHR individuals (Bodatsch et al., Reference Bodatsch, Ruhrmann, Wagner, Muller, Schultze-Lutter, Frommann and Brockhaus-Dumke2011; Fujioka et al., Reference Fujioka, Kirihara, Koshiyama, Tada, Nagai, Usui and Kasai2020; Kim et al., Reference Kim, Lee, Yoon, Lee and Kwon2018; Perez et al., Reference Perez, Woods, Roach, Ford, McGlashan, Srihari and Mathalon2014). Therefore, dMMN has the potential to be a clinically efficient biomarker that forecasts prognostic trajectories across the course of psychotic disorder, which would aid in clinical decision making regarding interventions for patients with FEP and subjects at CHR for psychosis.
In the current study, we aimed to investigate whether dMMN amplitude could be a potential biomarker to predict poor prognostic outcomes in FEP and CHR individuals. We hypothesized that (1) dMMN is impaired in FEP and CHR participants compared to healthy controls (HCs); thus, dMMN may be a potential biomarker for prognosis prediction in early psychosis patients; (2) baseline dMMN is smaller in FEP patients who are treatment resistant than in patients who are not treatment resistant and is predictive of later treatment resistance; and (3) dMMN at baseline is reduced in CHR individuals who are not remitted or who transition to a psychotic disorder compared to individuals who are remitted or who have not transitioned after a minimum of 1 year from the baseline dMMN assessment, and dMMN is predictive of future nonremission from CHR status or transition to a psychotic disorder.
Methods
Participants
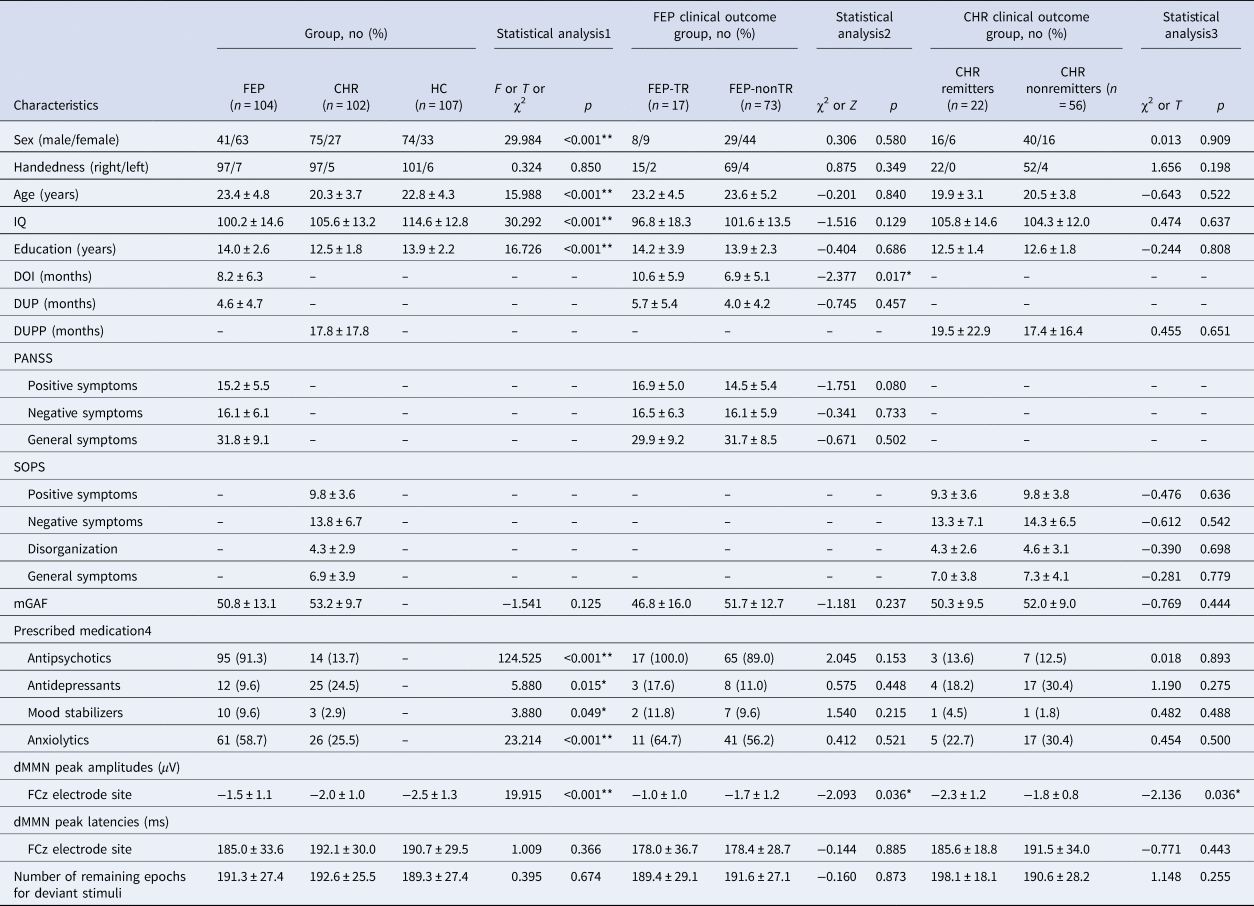
A total of 104 FEP patients, 102 individuals at CHR for psychosis, and 107 HCs participated in the baseline assessment, including dMMN recording, between August 2009 and June 2020. Among them, 25 FEP patients, 48 CHR subjects, and 47 HCs participated in our previous dMMN studies (Kim et al., Reference Kim, Cho, Yoon, Lee and Kwon2017, Reference Kim, Lee, Yoon, Lee and Kwon2018; Lho et al., Reference Lho, Kim, Lee, Kwak and Kwon2019). FEP patients and CHR individuals were recruited from the Seoul Youth Clinic (SYC; www.youthclinic.org), a center for early detection and intervention of psychosis (Kwon, Byun, Lee, & An, Reference Kwon, Byun, Lee and An2012) and from an inpatient and outpatient clinic of the Department of Neuropsychiatry at the Seoul National University Hospital (SNUH). The definition of FEP was an individual aged 16–40 years who satisfied the diagnosis of schizophreniform disorder, schizophrenia or schizoaffective disorder when assessed using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Axis I Disorders (SCID-I) and a duration of psychotic illness less than 2 years. Psychotic symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS). To confirm the CHR status of the participants, the Structured Interview for Prodromal Symptoms (SIPS) (Miller et al., Reference Miller, McGlashan, Rosen, Somjee, Markovich, Stein and Woods2002) was used. Prodromal symptoms were assessed using the validated Korean version of the Scale of Prodromal Symptoms (SOPS) (Jung et al., Reference Jung, Jang, Kang, Choi, Shin, Kim and Kwon2010; Miller et al., Reference Miller, McGlashan, Rosen, Cadenhead, Cannon, Ventura and Woods2003). In both the FEP and CHR groups, general functional status was defined using the modified Global Assessment of Functioning (mGAF) (Hall, Reference Hall1995). HCs were recruited via internet advertisement and were screened using the SCID-I Nonpatient Edition (SCID-NP). Potential HC participants were excluded if they had any first- to third-degree biological relatives with a psychotic disorder. Common exclusion criteria included substance abuse or dependence (except nicotine), neurological disease or significant head trauma, medical illness that could be accompanied by psychiatric symptoms, sensory impairments, and intellectual disability [intelligence quotient (IQ) < 70].
Among the 104 FEP patients, 90 patients with FEP received usual treatment, including antipsychotic medication, for at least 18 months until June 2020. Treatment resistance was defined when a patient showed at least moderately severe psychotic symptoms despite taking a sufficient dose (⩾20 mg olanzapine equivalent dose per day) of more than two antipsychotics for at least 12 months or taking clozapine according to the minimum requirement criteria of the Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines (Conley & Buchanan, Reference Conley and Buchanan1997; Gardner, Murphy, O'Donnell, Centorrino, & Baldessarini, Reference Gardner, Murphy, O'Donnell, Centorrino and Baldessarini2010; Howes et al., Reference Howes, McCutcheon, Agid, de Bartolomeis, van Beveren, Birnbaum and Correll2017). Certified psychiatrists who were blinded to the dMMN amplitudes thoroughly reviewed medical records to assess the symptom severity, medication status, and treatment adherence information provided by patients themselves and their caregivers at each visit to clinic. As a result, FEP patients were divided into treatment-resistant (FEP-TR, n = 17) and nonresistant (FEP-nonTR, n = 73) groups. The means and standard deviations of follow-up duration were 39.4 ± 32.7 months in the FEP-TR group and 46.1 ± 34.2 months in the FEP-nonTR group. Among the 102 CHR subjects, 78 CHR individuals participated in annual follow-up clinical assessment at least once until June 2020. A remitter was defined as a CHR individual who scored <3 on the SOPS positive symptoms subscale and 65⩾ on the mGAF measured at the last clinical assessment to reflect both symptomatic and functional recovery (Bossong et al., Reference Bossong, Antoniades, Azis, Samson, Quinn, Bonoldi and McGuire2019; Fujioka et al., Reference Fujioka, Kirihara, Koshiyama, Tada, Nagai, Usui and Kasai2020; Hamilton et al., Reference Hamilton, Roach, Bachman, Belger, Carrion, Duncan and Mathalon2019; Kim et al., Reference Kim, Lee, Yoon, Lee and Kwon2018). According to the remission criteria, certified psychiatrists who were blinded to dMMN amplitude determined 22 CHR remitters and 56 nonremitters, including 15 subjects who ended the follow-up assessment with a transition to a psychotic disorder (12 schizophrenia and three schizoaffective disorder) according to SIPS criteria. The means and standard deviations of follow-up duration were 46.0 ± 36.5 months for CHR remitters and 30.1 ± 27.9 months for CHR nonremitters. Sixty-three CHR individuals did not transition to psychotic disorder (online Fig. S1 in the Supplementary Material).
Written informed consent was obtained from all participants after they were given a thorough explanation of the study procedure (IRB no. H-1110-009-380). For minors, informed consent was obtained from both the participants themselves and their parents. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of SNUH (IRB no. H-2008-195-1154).
MMN acquisition
Continuous electroencephalographic (EEG) recording was conducted using a Neuroscan 128 Channel SynAmps system equipped with a 128-channel Quick-Cap based on the modified 10–20 international system (Compumedics, Charlotte, NC, USA) while participants were performing a passive auditory oddball task. The EEG data were digitized at a 1000 Hz sampling rate, and an online bandpass filter between 0.05 and 100 Hz was used. The reference electrodes were placed on both mastoids. The vertical and horizontal electrooculograms were recorded using electrodes below and on the outer canthus of the left eye to monitor eye movement artifacts. The resistance of all electrode sites was less than 5 kΩ. During the passive auditory oddball task performance, participants were instructed to ignore the auditory sound and concentrate on a ‘Where's Waldo?’ picture book. A pseudorandom series of 1000 Hz (80 dB, 10 ms rise/fall) auditory stimuli were binaurally presented using a STIM2 sound generator (Compumedics). With an intertrial interval of 600 ms, the duration of deviant stimuli was 100 ms (18.2%, 218/1200), and the duration of standard stimuli was 50 ms (81.8%, 982/1200).
Data preprocessing
Curry version 7 software (Compumedics) was used to preprocess the ERP data. After replacing bad channels using the linear interpolation of the adjacent channels (up to 7%), eye movement artifact reduction was performed according to the validated ocular artifact reduction algorithm (Semlitsch, Anderer, Schuster, & Presslich, Reference Semlitsch, Anderer, Schuster and Presslich1986). EEG recordings were rereferenced to common average reference data, and a 0.1–30 Hz bandpass filter was applied. Continuous EEG data were epoched to a 100 ms prestimulus interval and a 300 ms poststimulus interval, and the averaged prestimulus interval voltage was used in baseline correction. Automatic artifact rejection was performed by removing the epochs containing EEG amplitudes that exceeded ± 75 μV. The means and standard deviations of the numbers of remaining epochs for deviant stimuli were not significantly different across all groups (Table 1). dMMN was calculated by subtracting the ERPs elicited by the standard stimuli from those elicited by deviant stimuli. The most negative deflection between 130 and 250 ms poststimulus onset at the FCz electrode site, where dMMN had the maximal amplitude (Garrido, Kilner, Stephan, & Friston, Reference Garrido, Kilner, Stephan and Friston2009), was detected as the peak dMMN amplitudes and latencies.
Table 1. Demographic, clinical, and duration deviant mismatch negativity (dMMN) characteristics of the participants at baseline
FEP, first-episode psychosis; CHR, clinical high risk; HC, healthy control; FEP-TR, FEP patients who were treatment resistant; FEP-nonTR, FEP patients who were not treatment resistant; IQ, intelligence quotient; DOI, duration of illness; DUP, duration of untreated psychosis; DUPP, duration of untreated prodromal psychosis; PANSS, Positive and Negative Syndrome Scale; SOPS, Scale of Prodromal Symptoms; mGAF, modified Global Assessment of Functioning.
Data are indicated as the mean ± standard deviation.
*Statistical significance at p < 0.05.
**Statistical significance at p < 0.005.
1 Analysis of variance, independent t test or Welch's t test if the variances were not equal; χ2 analysis or Fisher's exact test for categorical data.
2 Mann–Whitney U test, χ2 analysis or Fisher's exact test for categorical data.
3 Independent t test or Welch's t test if the variances were not equal; χ2 analysis or Fisher's exact test for categorical data.
4 Number (percentage) of participants who were prescribed each medication at the time of dMMN measurement.
Statistical analysis
The demographic, clinical, and dMMN characteristics were compared using analysis of variance (ANOVA) or independent t test across the groups for continuous variables. A Bonferroni test was used for post hoc analysis. Welch's t test was performed if the variances were not equal, and Mann–Whitney U tests were used if the normality assumption was not satisfied. Chi-square tests were used for categorical variables. Group comparisons of MMN amplitudes and latencies across the FEP patients, CHR subjects, and HCs were performed using univariate analysis of covariance (ANCOVA) with age as a covariate. A post hoc simple contrast test was used to reveal specific group differences. Binary logistic regression analyses with the backward selection method were used to investigate whether baseline dMMN amplitudes were predictive of treatment resistance in FEP patients and predictive of nonremission or transition to psychotic disorder in CHR individuals. Common independent variables were the dMMN amplitude at FCz electrode site, sex, handedness, age, IQ, and education years in both FEP and CHR groups. Variables with significant group differences at baseline were selected as additional independent variables, which included the duration of illness (DOI) for FEP treatment resistance prediction and the SOPS positive subscale score at baseline for CHR transition prediction. Statistical analyses were performed using SPSS v.25.0 (IBM, Armonk, NY), and statistical significance was set at p < 0.05.
Results
Participant characteristics
Table 1 summarizes the demographic and clinical characteristics of the participants at baseline. Detailed information including follow-up characteristics of the clinical outcome groups is presented in online Supplementary Table S1 (FEP-TR v. FEP-nonTR) and online Supplementary Table S2 (CHR remitters v. CHR nonremitters and transitioned CHR v. nontransitioned CHR) in the Supplementary Material. There were more females than males in the FEP group than in the CHR and HC groups (χ2 = 29.984, p < 0.001). Individuals at CHR for psychosis were significantly younger and less educated than FEP patients (age, p < 0.001; education years, p < 0.001) and HCs (age, p < 0.001; education years, p < 0.001). IQ was highest in HCs (HC v. FEP, p < 0.001; HC v. CHR, p < 0.001) and lowest in FEP patients (FEP v. CHR, p = 0.014). In the clinical outcome group comparison, FEP-TR patients had a longer DOI than FEP-nonTR patients (Z = −2.377, p = 0.017), and transitioned CHR subjects had higher scores on the SOPS positive symptoms subscale (Z = −2.018, p = 0.044) than nontransitioned CHR individuals at baseline. Other baseline demographic and clinical characteristics, as well as follow-up duration, were not significantly different across the clinical outcome groups. Online Tables S3 and S4 in the Supplementary Material show no significant difference in baseline participant characteristics between the FEP patients who were assessed as treatment resistant and those who were not, as well as between CHR subjects who participated in the follow-up assessment and those who did not.
MMN characteristics
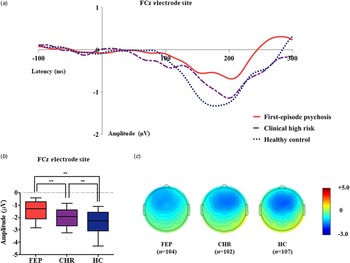
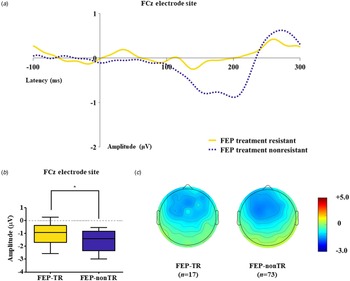
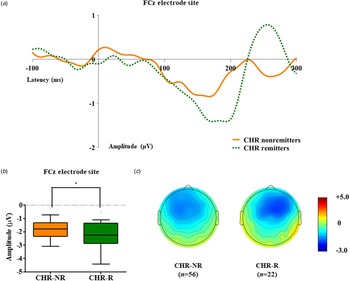
Table 1 presents group comparison results of baseline dMMN peak amplitudes and latencies at the FCz electrode site. ANCOVA with age as a covariate revealed a significant group difference in dMMN amplitude at the FCz electrode site (F = 19.915, p < 0.001) across the FEP, CHR, and HC groups. A post hoc simple contrast test showed that dMMN amplitudes were greatest in HCs, intermediate in CHR subjects, and smallest in FEP patients (FEP v. HC, p < 0.001; CHR v. HC, p = 0.002; FEP v. CHR, p = 0.004; Fig. 1). There was no significant group effect of dMMN peak latency. According to the clinical outcome groups, the FEP-TR group showed smaller dMMN amplitudes than the FEP-nonTR group (Z = −2.093, p = 0.036, Cohen's d = 0.634; Fig. 2), and dMMN amplitudes of CHR remitters were greater than those of CHR nonremitters (t = −2.136, p = 0.036, Cohen's d = 0.544; Fig. 3). In addition, ANCOVA with age as a covariate showed that dMMN amplitudes were significantly different across FEP patients, CHR nonremitters, CHR remitters, and HCs (F = 14.435, p < 0.001). A post hoc simple contrast test revealed that dMMN amplitudes were similarly impaired in FEP patients and CHR nonremitters compared to CHR remitters and HCs (FEP v. CHR nonremitters, p = 0.164; FEP v. CHR remitters, p = 0.004; CHR remitters v. HC, p = 0.441; online Fig. S2 in the Supplementary Material). Group comparison results across the FEP-TR group, FEP-nonTR group, CHR nonremitters, CHR remitters, and HCs are provided in the online Supplementary Material (Fig. S3). No significant group difference in dMMN amplitude was found between CHR subjects who transitioned to a psychotic disorder and those who did not (online Table S5 in the Supplementary Material). Online Tables S6 and S7 in the Supplementary Material show that there was no significant difference in dMMN characteristics between FEP patients who were assessed as treatment resistant and those who were not or between CHR subjects who participated in the follow-up assessment and those who did not.

Fig. 1. (a) Grand-averaged duration deviant mismatch negativity (dMMN) waveforms across the patients with first-episode psychosis (FEP), subjects at clinical high risk (CHR) for psychosis, and healthy controls (HCs) at the FCz electrode site. (b) dMMN amplitude across the groups at the FCz electrode site. Horizontal lines in groups indicate means, and vertical lines indicate 95% confidence intervals. *Indicates statistical significance at p < 0.05. **Indicates statistical significance at p < 0.005. (c) Two-dimensional topographic maps of dMMN in FEP patients, CHR individuals, and HCs. The colored bar with numbers indicates the dMMN amplitude (μV).

Fig. 2. (a) Grand-averaged duration deviant mismatch negativity (dMMN) waveforms across the patients with first-episode psychosis (FEP) who were treatment resistant (FEP-TR) and those who were not (FEP-nonTR) at the FCz electrode site. (b) dMMN amplitude across the clinical outcome groups at the FCz electrode site. Horizontal lines in groups indicate means, and vertical lines indicate 95% confidence intervals. *Indicates statistical significance at p < 0.05. (c) Two-dimensional topographic maps of dMMN in FEP-TR and FEP-nonTR patients. The colored bar with numbers indicates the amplitude of the dMMN (μV).

Fig. 3. (a) Grand-averaged duration deviant mismatch negativity (dMMN) waveforms across the individuals at clinical high risk (CHR) for psychosis who were remitted and those who were not at the FCz electrode site. (b) dMMN amplitude across the clinical outcome groups at the FCz electrode site. Horizontal lines in groups indicate means, and vertical lines indicate 95% confidence intervals. *Indicates statistical significance at p < 0.05. (c) Two-dimensional topographic maps of dMMN in CHR remitters and CHR nonremitters. The colored bar with numbers indicates the amplitude of the dMMN (μV).
Predicting prognostic trajectories using MMN
Table 2 presents the results of binary logistic regression analysis with the backward selection method. Treatment resistance in FEP was predicted by the dMMN amplitude [Exp(β) = 2.100, 95% confidence interval (95% CI) 1.104–3.993, p = 0.024] and DOI [Exp(β) = 1.138, 95% CI 1.031–1.256, p = 0.010]. Nonremission in CHR patients was predicted by the dMMN amplitude [Exp(β) = 1.895, 95% CI 1.065–3.374, p = 0.030]. The binary logistic regression model for predicting the transition to a psychotic disorder in CHR subjects did not include the dMMN amplitude but only included the SOPS positive subscale score at baseline [Exp(β) = 1.178, 95% CI 1.005–1.380, p = 0.043].
Table 2. Significant predictors of treatment resistance in first-episode psychosis (FEP) patients and nonremission or transition to psychotic disorder in subjects at clinical high risk (CHR) for psychosis in binary logistic regression analysis with the backward selection method
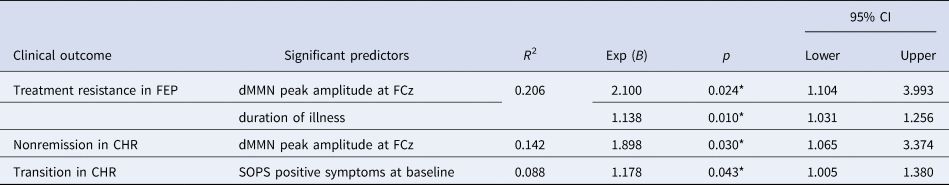
CI, confidence interval; dMMN, duration deviant mismatch negativity; SOPS, Scale of Prodromal Symptoms.
*Statistical significance, p < 0.05.
Discussion
Diagnosis based on the symptomatic phenotype produces significant prognostic heterogeneity that interferes with the goal of early intervention in early psychosis; thus, investigation of biomarkers that are predictive of prognostic trajectories of early psychosis patients is warranted (Allswede et al., Reference Allswede, Addington, Bearden, Cadenhead, Cornblatt, Mathalon and Cannon2020; Birchwood et al., Reference Birchwood, Todd and Jackson1998; Clementz et al., Reference Clementz, Sweeney, Hamm, Ivleva, Ethridge, Pearlson and Tamminga2016). In this longitudinal study, we aimed to confirm the usefulness of dMMN as a common prognostic biomarker across the early psychosis periods, from CHR to FEP. In line with many previous studies (Erickson et al., Reference Erickson, Ruffle and Gold2016; Higgins et al., Reference Higgins, Lewandowski, Liukasemsarn and Hall2021; Tateno et al., Reference Tateno, Higuchi, Nakajima, Sasabayashi, Nakamura, Ueno and Suzuki2021), dMMN amplitudes at the FCz electrode site were impaired in both FEP and CHR patients; thus, these amplitudes were used for further analysis for prognosis prediction. From the baseline assessment, dMMN amplitude was smaller in FEP-TR patients than in FEP-nonTR patients and was predictive of later treatment resistance. In addition, the dMMN amplitude was reduced in CHR nonremitters compared to CHR remitters at baseline and was associated with nonremission from a CHR status. These findings not only support the need for biomarkers predictive of prognostic trajectories but also highlight the usefulness of dMMN as a common prognostic biomarker across the early psychosis periods.
In the current study, impaired dMMN amplitude was observed from the baseline in FEP patients with poor prognosis (i.e. FEP-TR) and was a significant predictor of later treatment resistance. Considering that MMN generation is closely related to NMDA receptor-mediated glutamatergic activity (Javitt et al., Reference Javitt, Steinschneider, Schroeder and Arezzo1996; Uno & Coyle, Reference Uno and Coyle2019), the current study result is in line with previous studies that reported an abnormal NMDA-glutamate system and its association with poor treatment response in FEP patients (Dempster et al., Reference Dempster, Jeon, MacKinley, Williamson, Theberge and Palaniyappan2020; Mouchlianitis et al., Reference Mouchlianitis, Bloomfield, Law, Beck, Selvaraj, Rasquinha and Howes2016). The present study suggests that FEP patients with reduced dMMN amplitude may benefit from the early use of clozapine or adjuvant use of drugs targeting the NMDA-glutamate system (Rapado-Castro et al., Reference Rapado-Castro, Dodd, Bush, Malhi, Skvarc, On and Dean2017; Swerdlow et al., Reference Swerdlow, Bhakta, Chou, Talledo, Balvaneda and Light2016; Zheng et al., Reference Zheng, Zhang, Cai, Yang, Qiu, Ungvari and Xiang2018). In addition, recent studies have shown that treatment resistance to antipsychotics is evident upon illness onset in most FEP-TR patients (Demjaha et al., Reference Demjaha, Lappin, Stahl, Patel, MacCabe, Howes and Murray2017; Lally et al., Reference Lally, Ajnakina, Di Forti, Trotta, Demjaha, Kolliakou and Murray2016), suggesting that FEP treatment resistance can be forecasted by biomarkers showing abnormalities from the early psychosis period, such as MMN.
We found that CHR nonremitters presented a reduced dMMN amplitude, which was comparable to that of FEP patients and was smaller than that of HCs, whereas CHR remitters showed a similar magnitude of dMMN amplitude as that of HCs, which was larger than that of FEP patients. This finding supports the biological heterogeneity of CHR individuals, which may be a cause of the smaller effect size of MMN impairment found in the entire CHR group compared to FEP and schizophrenia patients (Erickson et al., Reference Erickson, Ruffle and Gold2016; Kim et al., Reference Kim, Cho, Yoon, Lee and Kwon2017). Furthermore, we replicated our previous report with extended cohort data and showed that a relatively preserved dMMN amplitude to a level of HCs at baseline was associated with a better clinical outcome, such as remission (Kim et al., Reference Kim, Lee, Yoon, Lee and Kwon2018); a similar result was provided by a recent study by Fujioka et al. (Reference Fujioka, Kirihara, Koshiyama, Tada, Nagai, Usui and Kasai2020). However, our analysis did not replicate previous study results that reported that impaired MMN was predictive of later transition to psychotic disorder (Bodatsch et al., Reference Bodatsch, Ruhrmann, Wagner, Muller, Schultze-Lutter, Frommann and Brockhaus-Dumke2011; Perez et al., Reference Perez, Woods, Roach, Ford, McGlashan, Srihari and Mathalon2014). The relatively small number of transitioned CHR subjects and the prescription of adequate medication, which aims to reduce the transition rate, may be the cause of the insignificant results of the current study. Therefore, future large-scale multisite longitudinal studies controlling for medication are warranted to test whether MMN could predict the prognosis of CHR individuals, including remission, nonremission, and transition to psychotic disorder, as in the recent study by Hamilton et al. (Reference Hamilton, Roach, Bachman, Belger, Carrion, Duncan and Mathalon2019) of auditory P300.
Although treatment guidelines for schizophrenia recommend the use of clozapine in patients who are resistant to usual antipsychotic treatment (Addington, Addington, Abidi, Raedler, & Remington, Reference Addington, Addington, Abidi, Raedler and Remington2017), it is difficult to know which FEP patients will be treatment resistant without spending a significant amount of time and resources and without extending patients’ suffering during trial and error. Moreover, in subjects at CHR for psychosis, although deciding the timing and intensity of intervention is more critical due to the nonspecific, heterogeneous, and changing nature of at-risk states, it is not easy to determine when to provide an intervention, which patients should receive an intervention, and the most suitable type of intervention to use due to the uncertain, diverse clinical outcomes of subjects at CHR for psychosis (Fusar-Poli et al., Reference Fusar-Poli, de Pablo, Correll, Meyer-Lindenberg, Millan, Borgwardt and Arango2020). In addition, existing studies proposing CHR risk calculators based on clinical and neurocognitive features suggest the importance of the incorporation of biomarkers, such as ERP components reflective of early psychosis states, to enhance model performance (Cannon et al., Reference Cannon, Yu, Addington, Bearden, Cadenhead, Cornblatt and Kattan2016; Oribe et al., Reference Oribe, Hirano, Del Re, Mesholam-Gately, Woodberry, Ueno and Niznikiewicz2020; Park et al., Reference Park, Lho, Hwang, Moon, Oh, Kim and Kwon2021; Schmidt et al., Reference Schmidt, Cappucciati, Radua, Rutigliano, Rocchetti, Dell'Osso and Fusar-Poli2017). Given that early psychosis periods exhibit a continuum of prodrome to FEP states, which share the common aspects of improved clinical outcomes by early interventions (Correll et al., Reference Correll, Galling, Pawar, Krivko, Bonetto, Ruggeri and Kane2018; Fusar-Poli et al., Reference Fusar-Poli, McGorry and Kane2017; Lieberman et al., Reference Lieberman, Perkins, Belger, Chakos, Jarskog, Boteva and Gilmore2001), common prognostic biomarkers that forecast prognosis across the early psychosis periods would provide valuable information for early intervention. In this regard, this study first suggested that MMN may serve as a common prognostic biomarker that should be included in future prediction model development to forecast clinical outcomes of early psychosis patients from CHR to FEP states.
This study has several limitations. First, medication use at the time of dMMN recording and the time to clinical outcome varied among FEP patients and CHR individuals; however, medication use at baseline and follow-up duration between clinical outcome groups were not different. Second, treatment resistance and nonremission were determined at a single last assessment that could not reflect changes outside the assessment points; thus, the findings should be interpreted with caution and potential biases should be considered. For example, a CHR individual who was classified as a remitter at the last follow-up assessment could subsequently become a nonremitter or transition to a psychotic disorder. Third, we used dMMN exclusively, unlike previous studies that used frequency-deviant MMN or double-deviant MMN (Bodatsch et al., Reference Bodatsch, Ruhrmann, Wagner, Muller, Schultze-Lutter, Frommann and Brockhaus-Dumke2011; Perez et al., Reference Perez, Woods, Roach, Ford, McGlashan, Srihari and Mathalon2014), which may be one of the causes of the negative results in the exploratory analysis for predicting the transition to psychotic disorder. Fourth, our sample size and the number of FEP-TR and transitioned CHR patients were small compared to other multisite studies, which may have diluted the effect of MMN on prognosis prediction. Considering that our sample was obtained from a single center (i.e. SYC), the sample size was relatively large, and our results are free from the issue of multicenter data stability. Nevertheless, large-scale multisite longitudinal studies with various MMN paradigms should be conducted to confirm the results of the current study.
To our knowledge, the present study is the first to investigate whether dMMN could serve as a common prognostic biomarker in early psychosis patients across the CHR to FEP. We observed that a reduced dMMN amplitude was already present at the baseline in poor clinical outcome groups and was predictive of treatment resistance in FEP patients and nonremission in subjects at CHR for psychosis. The present study suggests that reduced dMMN amplitude may support the clinical decision of early clozapine use for expected FEP-TR and the provision of rigorous treatment for potential CHR nonremission cases. In conclusion, the current study results provide information regarding dMMN as a common prognostic biomarker that could be added to existing prediction models based on clinical and neurocognitive features across early psychosis periods, which will aid in clinical decision-making for early interventions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721003068
Financial support
This research was supported by the Brain Research Program and the Basic Science Research Program through the National Research Foundation of Korea (NRF) and by the KBRI Basic Research Program through Korea Brain Research Institute, funded by the Ministry of Science, ICT & Future Planning (grant nos. 2017M3C7A1029610, 2019R1C1C1002457, 2020M3E5D9079910, and 21-BR-03-01).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.