Abstract
Background and objective
Escitalopram is one of the most commonly prescribed selective serotonin reuptake inhibitors (SSRIs). It is thought to act by blocking the serotonin transporter (SERT). However, its dose–SERT occupancy relationship is not well known, so it is not clear what level of SERT blockade is achieved by currently approved doses.
Methods
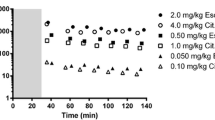
To determine the dose–occupancy relationship, we measured serial SERT occupancy using [11C]DASB [3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile] positron emission tomography (PET) and plasma drug concentrations after the administration of escitalopram in 12 healthy volunteers. We then built a pharmacokinetic–pharmacodynamic model to characterize the dose–occupancy relationship in the putamen and the dorsal raphe nucleus.
Results
Escitalopram at approved doses occupied less SERT than expected and the SERT occupancy showed regional effects [occupancy was higher in the dorsal raphe nucleus than in the putamen (p < 0.001)]. The drug concentration when 50 % of receptors are occupied (EC50) value and Hill coefficient were significantly different between the putamen (EC50 4.30, Hill coefficient 0.459) and the dorsal raphe nucleus (EC50 2.89, Hill coefficient 0.817).
Conclusions
Higher doses of escitalopram than 20 mg are needed to achieve 80 % or greater SERT occupancy. Higher occupancy by escitalopram in the dorsal raphe nucleus relative to the striatum may explain the delayed onset of action of SSRIs by modulating autoreceptor function. The prevention of the 5-HT1A autoreceptor-mediated negative feedback could be a strategy for accelerating the clinical antidepressant effects.








Similar content being viewed by others
References
Baldwin DS, Reines EH, Guiton C, Weiller E. Escitalopram therapy for major depression and anxiety disorders. Ann Pharmacother. 2007;41:1583–92.
Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–58.
Wade AG, Crawford GM, Yellowlees A. Efficacy, safety and tolerability of escitalopram in doses up to 50 mg in Major Depressive Disorder (MDD): an open-label, pilot study. BMC Psychiatry. 2011;11:42.
Dell’osso B, Arici C, Dobrea C, Camuri G, Benatti B, Altamura AC. Escitalopram tolerability as mono- versus augmentative therapy in patients with affective disorders: a naturalistic study. Neuropsychiatr Dis Treat. 2013;9:205–9.
Shim G, Park HY, Jang JH, Kim E, Park HY, Hwang JY, et al. What is the optimal dose of escitalopram for the treatment of obsessive-compulsive disorder? A naturalistic open-label study. Int Clin Psychopharmacol. 2011;26:284–90.
Guerdjikova AI, McElroy SL, Kotwal R, Welge JA, Nelson E, Lake K, et al. High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial. Hum Psychopharmacol. 2008;23:1–11.
Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–35.
Owens MJ, Krulewicz S, Simon JS, Sheehan DV, Thase ME, Carpenter DJ, et al. Estimates of serotonin and norepinephrine transporter inhibition in depressed patients treated with paroxetine or venlafaxine. Neuropsychopharmacology. 2008;33:3201–12.
Selvaraj S, Turkheimer F, Rosso L, Faulkner P, Mouchlianitis E, Roiser JP, et al. Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol Psychiatry. 2012;17:1254–60.
Kim E, Howes OD, Kim BH, Jeong JM, Lee JS, Jang IJ, et al. Predicting brain occupancy from plasma levels using PET: superiority of combining pharmacokinetics with pharmacodynamics while modeling the relationship. J Cereb Blood Flow Metab. 2012;32:759–68.
First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition: SCID-I/P, research. NYSPIDoB; 2002.
Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–9.
Lundberg J, Christophersen JS, Petersen KB, Loft H, Halldin C, Farde L. PET measurement of serotonin transporter occupancy: a comparison of escitalopram and citalopram. Int J Neuropsychopharmacol. 2007;10:777–85.
Kang KW, Lee DS, Cho JH, Lee JS, Yeo JS, Lee SK, et al. Quantification of F-18 FDG PET images in temporal lobe epilepsy patients using probabilistic brain atlas. Neuroimage. 2001;14:1–6.
Lee JS, Lee DS. Analysis of functional brain images using population-based probabilistic atlas. Curr Med Imaging Rev. 2005;1:81–7.
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–112.
Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000;27:1719–22.
Parsey RV, Kent JM, Oquendo MA, Richards MC, Pratap M, Cooper TB, et al. Acute occupancy of brain serotonin transporter by sertraline as measured by [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;59:821–8.
Turkheimer FE, Selvaraj S, Hinz R, Murthy V, Bhagwagar Z, Grasby P, et al. Quantification of ligand PET studies using a reference region with a displaceable fraction: application to occupancy studies with [(11)C]-DASB as an example. J Cereb Blood Flow Metab. 2012;32:70–80.
Schoemaker RC, van Gerven JM, Cohen AF. Estimating potency for the Emax-model without attaining maximal effects. J Pharmacokinet Biopharm. 1998;26:581–93.
Alvan G, Paintaud G, Wakelkamp M. The efficiency concept in pharmacodynamics. Clin Pharmacokinet. 1999;36:375–89.
Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S. Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry. 2002;7:317–21.
Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–71.
Coleman JA, Green EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature. 2016;532:334–9.
Zeng Z, Chen TB, Miller PJ, Dean D, Tang YS, Sur C, et al. The serotonin transporter in rhesus monkey brain: comparison of DASB and citalopram binding sites. Nucl Med Biol. 2006;33:555–63.
Lundberg J, Tiger M, Landen M, Halldin C, Farde L. Serotonin transporter occupancy with TCAs and SSRIs: a PET study in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15:1167–72.
Baldinger P, Kranz GS, Haeusler D, Savli M, Spies M, Philippe C, et al. Regional differences in SERT occupancy after acute and prolonged SSRI intake investigated by brain PET. Neuroimage. 2014;88:252–62.
Hinz R, Selvaraj S, Murthy NV, Bhagwagar Z, Taylor M, Cowen PJ, et al. Effects of citalopram infusion on the serotonin transporter binding of [11C]DASB in healthy controls. J Cereb Blood Flow Metab. 2008;28:1478–90.
Sogaard B, Mengel H, Rao N, Larsen F. The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J Clin Pharmacol. 2005;45:1400–6.
Burke WJ. Escitalopram. Expert Opin Investig Drugs. 2002;11:1477–86.
Rabinowitz I, Baruch Y, Barak Y. High-dose escitalopram for the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol. 2008;23:49–53.
Dougherty DD, Jameson M, Deckersbach T, Loh R, Thompson-Hollands J, Jenike M, et al. Open-label study of high (30 mg) and moderate (20 mg) dose escitalopram for the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol. 2009;24:306–11.
Savli M, Bauer A, Mitterhauser M, Ding Y-S, Hahn A, Kroll T, et al. Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage. 2012;63:447–59.
Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–35.
Vauquelin G, Charlton SJ. Long-lasting target binding and rebinding as mechanisms to prolong in vivo drug action. Br J Pharmacol. 2010;161:488–508.
Gifford AN, Gatley SJ, Volkow ND. Evaluation of the importance of rebinding to receptors in slowing the approach to equilibrium of high-affinity PET and SPECT radiotracers. Synapse. 1998;28:167–75.
Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tissue input functions. J Cereb Blood Flow Metab. 2000;20:1111–33.
Malagie I, Trillat AC, Jacquot C, Gardier AM. Effects of acute fluoxetine on extracellular serotonin levels in the raphe: an in vivo microdialysis study. Eur J Pharmacol. 1995;286:213–7.
Gartside SE, Umbers V, Hajos M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–70.
Bel N, Artigas F. Fluvoxamine preferentially increases extracellular 5-hydroxytryptamine in the raphe nuclei: an in vivo microdialysis study. Eur J Pharmacol. 1992;229:101–3.
Nord M, Finnema SJ, Halldin C, Farde L. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol. 2013;16:1577–86.
Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152.
Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:342–8.
Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs. 2013;27:703–16.
Koch W, Unterrainer M, Xiong G, Bartenstein P, Diemling M, Varrone A, et al. Extrastriatal binding of [(1)(2)(3)I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014;41:1938–46.
Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–501.
Kim E, Howes OD, Park JW, Kim SN, Shin SA, Kim BH, et al. Altered serotonin transporter binding potential in patients with obsessive-compulsive disorder under escitalopram treatment: [11C]DASB PET study. Psychol Med. 2016;46:357–66.
Acknowledgments
We thank June Hee Lee, Seong A Shin and Boeun Lee for their kind assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (no. NRF-2015R1A5A7037676) and Grant No. 14-2014-007 from the SNUBH Research Fund. Dr. Oliver Howes was funded by the Medical Research Council-UK (no. MC-A656-5QD30), the Maudsley Charity (no. 666), the Brain and Behavior Research Foundation, Wellcome Trust (no. 094849/Z/10/Z) and the National Institute for Health Research (NIHR) Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London.
Conflicts of interest
Kim E. has participated in speaker meetings organized by Otsuka. Howes O.D. has received investigator-initiated research funding from, and/or participated in advisory/speaker meetings organized by, Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Kwon J.S. has received investigator-initiated research funding from Otsuka and has participated in advisory/speaker meetings organized by Jansenn, Otsuka and Dainippon Sumitomo Pharma. Kim B.-H., Chon M.-W., Turkheimer F.E., Lee J.S., Lee Y.-S., and Seo S. have no conflicts of interest. Neither the authors nor their families have been employed by, or have holdings/a financial stake in, any biomedical company.
Ethical approval
This study was conducted in observance with the Declaration of Helsinki after the study protocol was approved by the Institutional Review Board of Seoul National University Hospital.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kim, E., Howes, O.D., Kim, BH. et al. Regional Differences in Serotonin Transporter Occupancy by Escitalopram: An [11C]DASB PK-PD Study. Clin Pharmacokinet 56, 371–381 (2017). https://doi.org/10.1007/s40262-016-0444-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0444-x